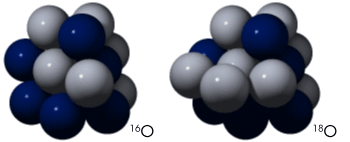
Oxygen is one of the most significant keys to deciphering past climates. Oxygen comes in heavy and light varieties, or isotopes, which are useful for paleoclimate research. Like all elements, oxygen is made up of a nucleus of protons and neutrons, surrounded by a cloud of electrons. All oxygen atoms have 8 protons, but the nucleus might contain 8, 9, or 10 neutrons. “Light” oxygen-16, with 8 protons and 8 neutrons, is the most common isotope found in nature, followed by much lesser amounts of “heavy” oxygen-18, with 8 protons and 10 neutrons. |
|||
The ratio (relative amount) of these two types of oxygen in water changes with the climate. By determining how the ratio of heavy and light oxygen in marine sediments, ice cores, or fossils is different from a universally accepted standard, scientists can learn something about climate changes that have occurred in the past. The standard scientists use for comparison is based on the ratio of oxygen isotopes in ocean water at a depth of 200-500 meters. What climate factors influence the ratio of oxygen isotopes in ocean water?Evaporation and condensation are the two processes that most influence the ratio of heavy oxygen to light oxygen in the oceans. Water molecules are made up of two hydrogen atoms and one oxygen atom. Water molecules containing light oxygen evaporate slightly more readily than water molecules containing a heavy oxygen atom. At the same time, water vapor molecules containing the heavy variety of oxygen condense more readily. |
The Oxygen-18 isotope has an extra two neutrons, for a total of 10 neutrons and 8 protons, compared to the 8 neutrons and 8 protons in a normal oxygen atom. The slighty greater mass of 18O—12.5 percent more than 16O—results in differentiation of the isotopes in the Earth’s atmosphere and hydrosphere. Scientists measure differences in oxygen isotope concentrations to reveal past climates. [Roll mouse over nuclei to animate.] (Illustration by Robert Simmon, NASA GSFC) | ||
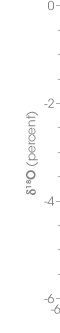 |
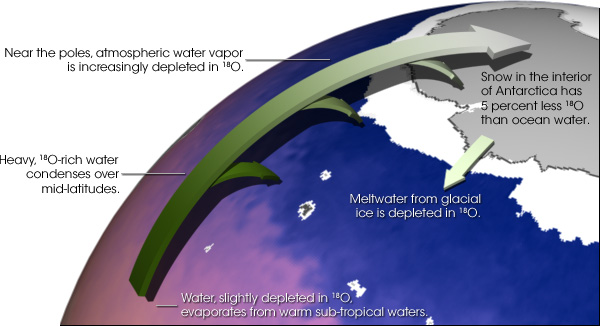
As air cools by rising into the atmosphere or moving toward the poles, moisture begins to condense and fall as precipitation. At first, the rain contains a higher ratio of water made of heavy oxygen, since those molecules condense more easily than water vapor containing light oxygen. The remaining moisture in the air becomes depleted of heavy oxygen as the air continues to move poleward into colder regions. As the moisture reaches the upper latitudes, the falling rain or snow is made up of more and more water molecules containing light oxygen. Ocean waters rich in heavy oxygen: During ice ages, cooler temperatures extend toward the equator, so the water vapor containing heavy oxygen rains out of the atmosphere at even lower latitudes than it does under milder conditions. The water vapor containing light oxygen moves toward the poles, eventually condenses, and falls onto the ice sheets where it stays. The water remaining in the ocean develops increasingly higher concentration of heavy oxygen compared to the universal standard, and the ice develops a higher concentration of light oxygen. Thus, high concentrations of heavy oxygen in the ocean tell scientists that light oxygen was trapped in the ice sheets. The exact oxygen ratios can show how much ice covered the Earth. Ocean waters rich in light oxygen: Conversely, as temperatures rise, ice sheets melt, and freshwater runs into the ocean. Melting returns light oxygen to the water, and reduces the salinity of the oceans worldwide. Higher-than-standard global concentrations of light oxygen in ocean water indicate that global temperatures have warmed, resulting in less global ice cover and less saline waters. Because water vapor containing heavy oxygen condenses and falls as rain before water vapor containing light oxygen, higher-than-standard local concentrations of light oxygen indicate that the watersheds draining into the sea in that region experienced heavy rains, producing more diluted waters. Thus, scientists associate lower levels of heavy oxygen (again, compared to the standard) with fresher water, which on a global scale indicates warmer temperatures and melting, and on a local scale indicates heavier rainfall. |
The concentration of 18O in precipitation decreases with temperature. This graph shows the difference in 18O concentration in annual precipitation compared to the average annual temperature at each site. The coldest sites, in locations such as Antartica and Greenland, have about 5 percent less 18O than ocean water. (Graph adapted from Jouzel et. al., 1994) | |
 | |||
Paleoclimatologists use oxygen ratios from water trapped in glaciers as well as the oxygen absorbed in the shells of marine plants and animals to measure past temperatures and rainfall. In polar ice cores, the measurement is relatively simple: less heavy oxygen in the frozen water means that temperatures were cooler. Oxygen isotopes in ice cores taken from mountain tops closer to the equator are more difficult to measure since heavy oxygen tends to fall near the equator regardless of temperature. In shells, the measurement is far more complicated because the biological and chemical processes that form the shells skew the oxygen ratio in different ways depending on temperature. Fossilized oxygen isotopesThe shells of tiny plants and animals and corals are typically made of calcium carbonate (CaCO3), which is the same as limestone, or chalk, or silicon dioxide (SiO2), similar to the compound common in quartz sand. As the shells form, they tend to incorporate more heavy oxygen than light oxygen, regardless of the oxygen ratio in the water. The biological and chemical processes that cause the shells to incorporate greater proportions of heavy oxygen become even more pronounced as the temperature drops, so that shells formed in cold waters have an even larger proportion of heavy oxygen than shells formed in warmer waters, where the difference is less notable. This temperature-based skew effect means that the oxygen isotope make-up of shells would not precisely match the make-up of the ocean water in which they grew. Scientists must correct for this skew if they are to learn about the ratio of oxygen isotopes in the ocean waters where the shells formed. |
Water vapor gradually loses 18O as it travels from the equator to the poles. Because water molecules with heavy 18O isotopes in them condense more easily than normal water molecules, air becomes progressively depleted in 18O as it travels to high latitudes and becomes colder and drier. In turn, the snow that forms most glacial ice is also depleted in 18O. As glacial ice melts, it returns 16O-rich fresh water to the ocean. Therefore, oxygen isotopes preserved in ocean sediments provide evidence for past ice ages and records of salinity. (Illustration by Robert Simmon, NASA GSFC) | ||
 |
 | ||
Correcting for the Skew: Scientists can correct for the temperature factor by looking at other chemicals in the shells. In coral, for example, the balance between strontium and calcium is determined by temperature. By measuring the amounts of these chemicals in the coral, scientists can determine the ocean’s temperature, then calculate how much more heavy oxygen the shells were likely to incorporate at that temperature. Discrepancies in the oxygen isotope ratio after the temperature correction reveal changes in the ocean’s local salinity, which is related to evaporation, rainfall and runoff, and global salinity—a measure of the total amount of ice in the world. The oxygen isotope ratio has the potential to tell scientists about past climate anywhere that the ratio is preserved in water chemistry or elsewhere. Scientists are moving forward to apply this powerful tool to more and more branches of paleoclimatology.
|
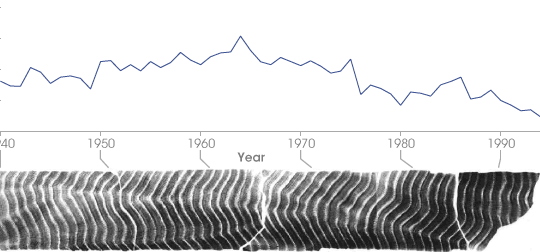
Corals with annual growth rings are extraordinarily useful to paleoclimatologists because they combine an oxygen-isotope record with precise dating. This x-ray of a coral core shows the change in 18O concentration corresponding to the coral’s growth. Because living organisms interact with their environment in complex ways, isotope measurements made from coral and other fossils must be carefully calibrated. (NASA figure by Robert Simmon, based on data provided by Cole et. al. 2000, archived at the World Data Center for Paleoclimatology) | ||